How does HER-096 work in the body?
The complex brain pathology underlying degeneration and death of dopamine neurons involves:
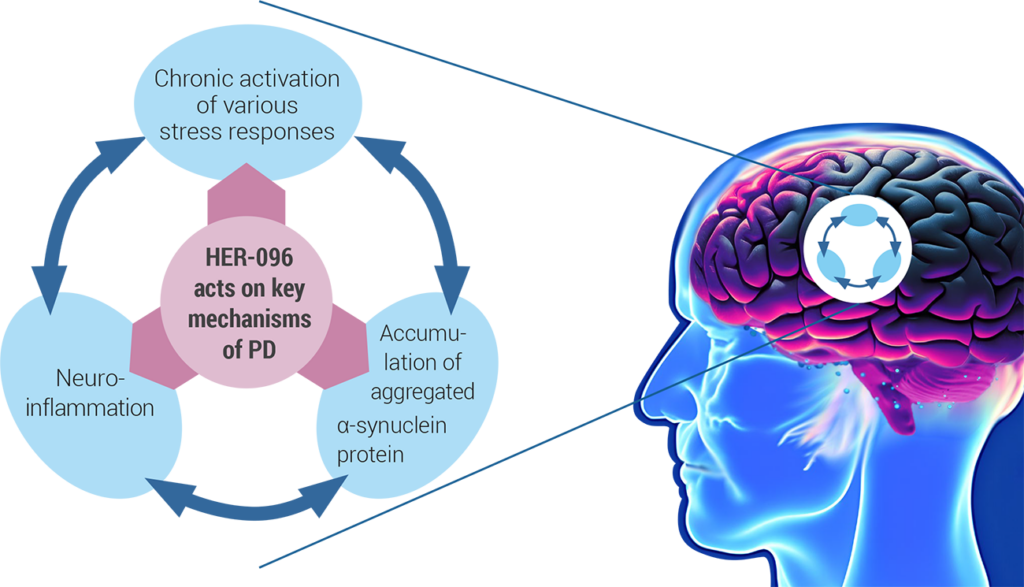
These different mechanistic aspects of the disease are intimately interlinked and may feed forward each other.
While the root cause of Parkinson’s disease still remains poorly understood, the therapeutic hypothesis of HER-096 is based on breaking this vicious cycle to protect dopamine neurons from further degeneration – and to promote their functional recovery.
HER-096 was developed based on the active site of cerebral dopamine neurotrophic factor (CDNF), an endogenous human protein that
- Regulates the UPR pathway.
- Protects neurons from cell death induced by, e.g., chronically elevated endoplasmic reticulum (ER) stress
HER-096 development is built on 15 years of research and development work related to CDNF’s role in neurodegeneration both in the academia and in the industry. This work has generated many important insights and data to help us understand how HER-096 works, and how cells and tissues respond to exposure to HER-096.
- HER-096 is different from CDNF as it can effectively pass the blood-brain barrier (BBB). This allows easy peripheral administration of HER-096 as opposed to the complicated intracranial delivery that is required for the CDNF protein.
- The combination of the disease-modifying potential of HER-096 with its capacity of BBB penetration makes it a compelling candidate to become a ground-breaking novel treatment for Parkinson’s disease, with potential for slowing down or stopping the disease process – not just treating the symptoms of the disease.
